Introduction:
Adult T cell leukemia/lymphoma (ATLL) is an aggressive mature T cell neoplasm caused by Human T lymphotropic virus type 1/2 (HTLV1/2) infection. HTLV1/2 is endemic in several geographic areas such as Latin America, Japan and intertropical Africa with estimated 5-10 million individuals infected worldwide. There is no standard therapy for ATLL and most patients are treated with regimens used for aggressive lymphomas. We hereby reviewed the outcome of patients treated in two US academic medical centers with a high prevalence of ATLL.
Methods:
We retrospectively evaluated all of the patients with a confirmed diagnosis of ATLL seen in two academic medical centers in Florida, USA. Baseline characteristics, treatments regimens and clinical outcomes where evaluated. First line therapies were categorized as treatment with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone), Intensive chemotherapy (HyperCVAD, VCAP-AMP-VECP, or EPOCH), vs others (Interferon and zidovudine, Gemcitabine Oxaliplatin, Pralatrexate, and Gemcitabine cisplatin dexamethasone). Patient and disease characteristics were compared using Chi-square. Progression free survival (PFS) and overall survival (OS) for different chemotherapy regimens were compared using log-rank testing.
Results
A total of 61 patients were included in the analysis. The median age was 58 (32-75) years. Two thirds of the patients were female. The majority were of African American descent (82.5%) (Table 1), and 35.6% were originally from Jamaica, followed by 13.6% from Haiti.
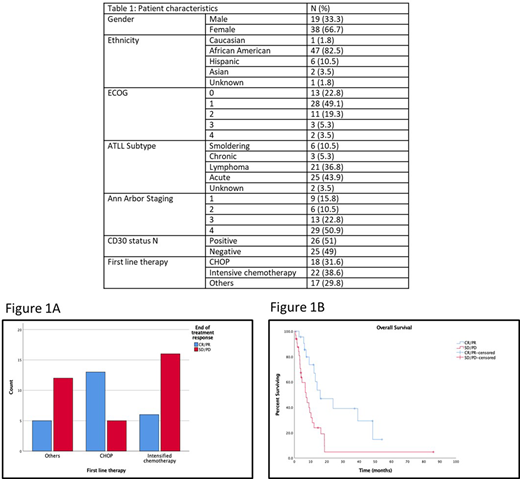
Patients were more likely to have CR/PR to CHOP (72%) compared to those treated with intensive chemotherapy or others (27.3% and 29.4% respectively) (p=0.007) (figure 1A). While there was no statistically difference in baseline characteristics in patients receiving the three categories of treatments (i.e. age, stage, ECOG, CD30 status), patients with acute subtype were less likely to be treated with CHOP (21.1% vs 47.4% respectively, p=0.096) which might explain the discrepancies in response. There was no statistical difference in PFS and OS between the three CHOP, Intensive chemotherapy and others (6.4 mo, 3.1 mo, 2.1 mo, p=0.23) and (14 mo, 8.9 mo, 18.5 mo, p=0.14) respectively. Patients responding to first line therapy (CR/PR) had improved OS when compared to those having stable/progressive disease (15.9 mo vs 7.2 mo) (p=0.004) (Figure 1B).
Conclusions:
Our study results reflect the poor outcomes of ATLL patients, especially in the ones with primary refractory disease to frontline treatments. We also showed the suboptimal activity of frontline regimens for ATLL and lack of unifying therapeutic approach for this lymphoma in the US. Multi-institutional clinical trials exploring novel therapeutic frontline options to improve response rates, which translate in better OS are desperately needed in this population.
Sandoval-Sus:Massive Bio: Consultancy; Janssen: Consultancy; MorphoSys US: Consultancy; Celgene: Speakers Bureau. Sokol:Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kyowa/Kirin Inc.: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal